11
See for yourself whether your answer was right:
| According to your answer | Actual result | ||
|---|---|---|---|
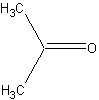 |
2 CH3 groups |
|
|
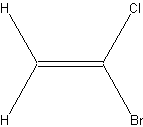 |
1 =CH2 group |
|
|
Apparently it is not significant how many groups there are that contain protons!
You assumed that there would be two signals in the case of acetone, the first compound. In other words, you think that the two methyl groups experience different chemical shifts. But that would mean that the electronic environment around the two methyl groups is different. Is that actually the case?
In the case of the second compound you would have expected to find only one signal since you assumed that both protons would have the same chemical shift. Do these hydrogens really have the same, spatially equivalent environment?
| Reconsider these questions and try to come up
with a better answer for the initial question. Go
back, do not pass go, do not collect $2000. |