67
Great! You've got the correct answer!
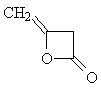
| The isomer |
|
is identical to the di-ketene in question. |
- D) gives rise to two NMR signals from the protons in the CH2= and >CH2 groups.
- The ratio of the intensities is 1 : 1, since both of the two groups contain two equivalent protons.