83
Determination of coupling constants:
The coupling constant JAX is defined as follows:JAX = Separation in Hertz of two neighboring lines of the multiplet A
However, the spectra are typically labelled in the d scale. To convert from the d scale to the n scale, the following relation holds:
| d = DnPr · 106 / n0 ppm |
Basically, you have to convert the separation of two neighboring lines in ppm to the difference in frequency JAX in Hertz.
A similar conversion between the different scales used to label NMR spectra was already covered on page 42!
An additional example:
A quartet appears in the NMR spectrum of
acetaldehyde CH3-CHO at d
= 9.26 ppm (Spectrometer frequency n0
= 300 MHz *). 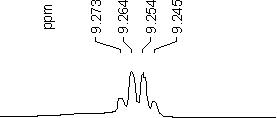
Compare your answer to the solution on page 79! * Spectrum courtesy of Pacific Lutheran University |