75
Answer:
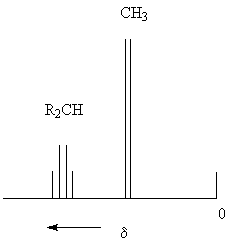
The appearance of a quartet for the R2CH proton can be explained as follows:
In the external magnetic field B0 the nuclear spin of the proton can either align itself either parallel or antiparallel to the external field. These two alignments are eqivalent to two different energy levels, between which transitions are possible. And these transitions give rise to the resonance signals.
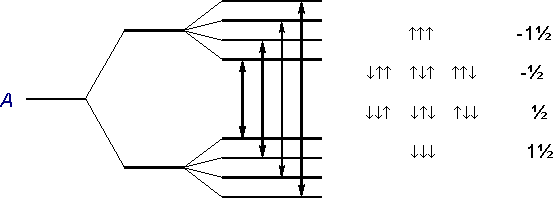
The protons in the neighboring CH3 group also align themselves with the external field. However, the individual spins of the three protons add up to the following total spins:
 |
!!! |
The magnetic fields of these total spins modify - mediated by the bond electrons - the external magnetic field B at the proton of the R2CH group, so that both of the two possible energy levels of H are split up into four levels. Therefore four different, closely spaced transitions are possible. (Quartet: nCH3 = 3, therefore MCH = 4).
With a similar approach you can determin the multiplicity of the signal from the CH3 group! (For additional examples, see pages 74 und 76.)
Does your line spectrum show additional differences? Check page 77!
You will come up with other characteristic
multiplets if you try to deduce if you try to deduce the line spectra
for the following molecules:
Use the same style as above, and compare your predictions with the official solutions on page 81 |